ACTRI Dashboards
The ACTRI Impact Dashboard highlights our commitment to advancing clinical and translational science and research through three key focus areas: Data Science and Support, Research Educational Programs, Pilot Studies, & Clinical Protocols.
Data Science and Support (DSS)
Data Science and Support (DSS)
The ACTRI DSS unit is led by Mike Hogarth, MD, Professor of Medicine and UM1 MPI. The DSS unit incorporates and improves access to data and computational expertise and resources across UCSD (e.g., Division of Biostatistics, School of Engineering, Department of Mathematics, Halıcıoğlu Data Science Institute) and specialized quantitative expertise and resources at La Jolla Institute for Immunology (LJI), Salk Institute, and Sanford Burnham Prebys (SBP).
Biostatistics, Epidemiology, and Research Design (BERD)
Lead: Lin Liu, PhD
Contact: actri-berd@health.ucsd.edu
BERD had a total of 203 visits across the past three years (2021-2023).
BERD annually supports over 120 pre- and post-award services, provides an average of 1,650 hours of post-award data analysis support to the ACTRI members, and an average of 70 walk-in statistical consultation visits.
Services include:
- The BERD Unit provides statistical support at all research phases
- Through pre-award services, BERD provides expertise on innovative study design and proposal writing which includes specifying, refining and developing statistical analysis plans, and performing sample size and power calculations. Additionally, BERD provides mentorship for K award applicants.
- Through post-award services, BERD performs various data analysis using conventional and advanced statistical methods such as: linear and generalized linear regression models, machine learning approaches, longitudinal data analysis, survival analysis (including competing risk and recurrent events), causal inference using the propensity score method, and resampling approaches. Moreover, BERD assists inwriting and reviewing manuscripts to ensure statistical works are properly represented.
- BERD provides guidance on statistical and research design to experienced and novice clients during weekly office hour. BERD experts also serve on the ACTRI Scientific Review Committee and review all pilot project and K applications.
Biomedical Informatics (BMI)
Lead: Mike Hogarth, MD
AI expert: John Ayers, PhD
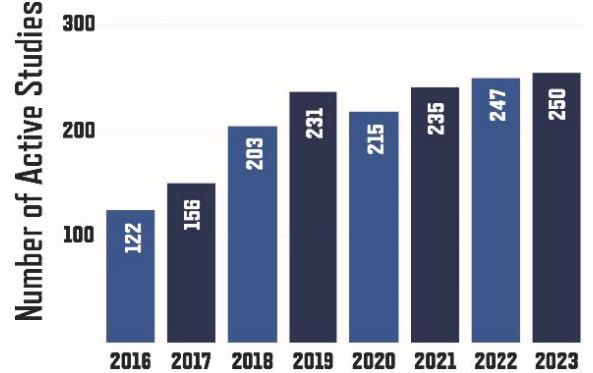
BMI manages 1.2 million records with 40 billion data points with an average of 1,800 annual requests. These activities have increased about 7% each year since 2020.
Annual Biomedical Informatics Requests. (n=1800)
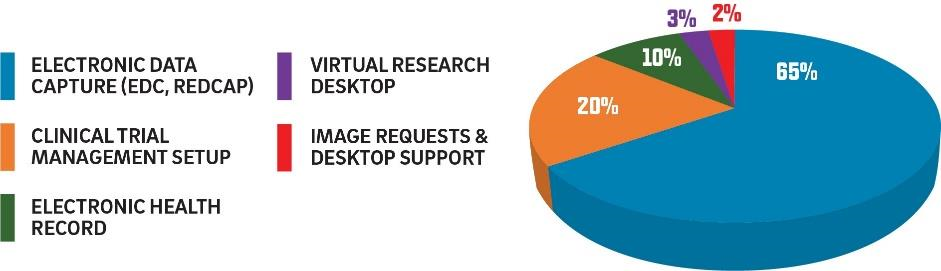
Services include:
- Consultations, support, and access to cutting-edge software, including a clinical trial management system (i.e., Velos), specialized research functions in the EHR (e.g., Epic Research, ACTRI Clinical Data Warehouse for Research), secure data enclaves (i.e., Health Secure Research Cloud), a scalable big data platform (Databricks), data extraction concierge services, natural language processing (NLP) pipelines, and input and support for multiple NCATS data platforms (e.g., ENACT, N3C).
- Broad range of electronic data capture (EDC) for research, including REDCap, mobile EDC through a secure decentralized trial (DCT) platform, and EDC within the EHR that pulls data using Fast Healthcare Interoperability Resources.
Center for Computational Biology & Bioinformatics (CCBB)
Lead: Kathleen Fisch, PhD
Contact: kfisch@health.ucsd.edu
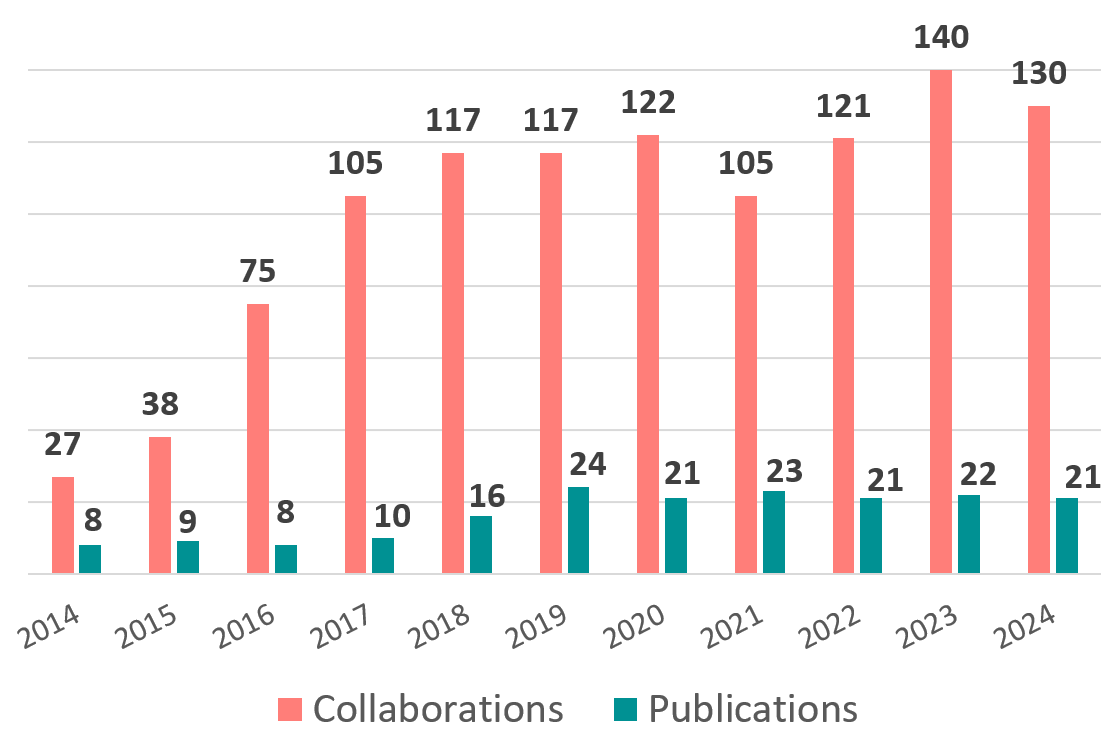
CCBB averages 120 consultations, supports 87 project requests, and collaborates on 60 grant applications annually.
CCBB co-authored 154 peer-reviewed papers since its inception in 2014, and on average, service requests have grown 26% annually since 2018 with a peak in 2021.
Services include:
- Study design consultation and analysis of large multi-omics datasets with an emphasis on systems biology, genomics, transcriptomics, epigenomics, single cell, and spatial technologies.
- CCBB leverages BMI’s secure scalable cloud computing environment to link genomics datasets with clinical data for research use.
CCBB Collaborations & Publications
Data Science and Support Training
Lead: Colin Depp, PhD
ACTRI staff host UCSD Health Data Day, an annual full-day symposium during which over 150 investigators and staff learn how to use new tools and data sets.
Research Programs
Research Programs
Our commitment to nurturing a Clinical and Translational Science (CTS) community that is both diverse and highly qualified is exemplified by our workforce development programs that have supported >2500 trainees and scholars across the career continuum since 2018.
SUSTAIN - Supporting Under-represented Scholars in Translational and Interdisciplinary Networks
Lead: Adriana Tremoulet, MD, MAS and Colin Depp, PhD
Contact: ACTRI-Education@health.ucsd.edu
Launched in 2020, SUSTAIN provides 1-2 years of protected time, individualized mentorship, and grant writing training.
5 out of our 6 SUSTAIN alumni, successfully transitioned to independently funded research.
Since 2018, UCSD has received three R25 programs that attract UR faculty nationwide and train >25 scholars from UR backgrounds per year. These R25 programs are co-led by Dr. JoAnn Trejo, WD’s Director of Career Development alongside other WD faculty, including Dr. Smith (UM1 MPI) who co-leads the R25 in infectious diseases.
UCSD was one of the seven institutions to receive an NIH Faculty Institutional Recruitment for Sustainable Transformation (FIRST) award, which provided support for 12 new faculty from UR backgrounds; this program is also co-led by Dr. Trejo.
Shared leadership and coordination among all these programs improved recruitment for diverse faculty, as now 31% of K applicants report UR backgrounds.
Medical Student Research Gap (MedGap) Year Program
Lead: H. Irene Su, MD MSCE
Contact: hisu@ucsd.edu
UCSD medical students may participate in this 12-month program between their 3rd and 4th years of medical school. Students in the program will identify a UCSD faculty research mentor, will work in their mentor's lab or research group, and engage in career development sessions with their cohort over the year.
Clinical Research Enhancement through Supplemental Training (CREST)
Lead: Ravindra L. Mehta, MD
Contact: Seble Chernet (schernet@ucsd.edu)
CREST offers flexible options: individual “a la carte” courses, a 10-course certificate, and a Master’s in Advanced Studies (MAS) degree. The program extends to hybrid, fully remote, and asynchronous delivery options.
All UCSD K scholars have enrolled in CREST, and the program is also accessed by non-UCSD partners at the ACTRI, clinical trial coordinators, regulatory and industry professionals.
650 learners since 2018.
158 learners have completed a Translational Science Track courses since 2018. This track immerses students in the translational process, guiding them from discovery to Phase I trials with didactics in the fundamentals of CTS, case studies in translation, and a team science application of learning to real-world challenges.
CreAting Diversity in Science through Research Education (CADRE)
Lead: Han Nguyen and Carlos Rojas, MBA
Starting in 2024, undergraduate students are matched with a translational research group and receive career skills training and work experience while being paid.
7 Mesa College students completed the research program in 2024.
Pilots
Pilots
Lead: Murray Stein, MD, MPH
Contacts: (Murray Stein, MD, MPH) mstein@health.ucsd.edu & (Kathleen Kennedy) kkennedy@health.ucsd.edu
Dr. Stein leads a team of over 60 faculty, nurses, and community members who review applications. He is supported by the Scientific Review Committee and all the ACTRI units in both rigorous scientific review of applications and mentoring of awardees.
Across ACTRI institutions, we previously received 35-70 applications per cycle (2018-2023) and funded around 10 awards per cycle.
ACTRI Pilot Grants Help Investigators Take-Off since 2018

The program has funded 68 projects that cover the translational continuum from basic science to dissemination and implementation over the past five years. Following their Pilot Award, the recipients received a total of 31 R01 or equivalent grants (not necessarily related to Pilot project) as their first post-Pilot award, accumulating a subsequent total of ~$48M (114 total grants and $79 M in total funding, three grants from NCATS, and over 1600 publications) clearly emphasizing the career success of these awardees post-pilot grant funding.
The ACTRI provided $1,800,000 in pilot project grants, leading to over 1600 publications and $48 million in new R01 or equivalent grants.
Galvanizing Engineering in Medicine (GEM)
An interdisciplinary pilot project program between the ACTRI and UCSD School of Engineering where clinicians and engineers develop innovative technologies that can be applied to solve challenging CTS problems in health care.
Since 2018, 32 GEM Projects, resulted in:
- 243 publications/poster presentations
- 32 patents
- 7 startup companies
- $204M in subsequent funding (>30% in COVID-related projects)
KL2 Program
KL2 Progam
The ACTRI KL2 Grant Support program is a research training grant for junior faculty, that provides up to three years of research career development support and up to $120,000 for UC San Diego instructors or assistant professors.
As of April 2024:
- 1588 Total KL2 publications
- 58.8 The average publications per graduate [sd=42.1]
- 100% of KL2 Graduates are still primarily in Active Research
Learn more about the KL2 Program
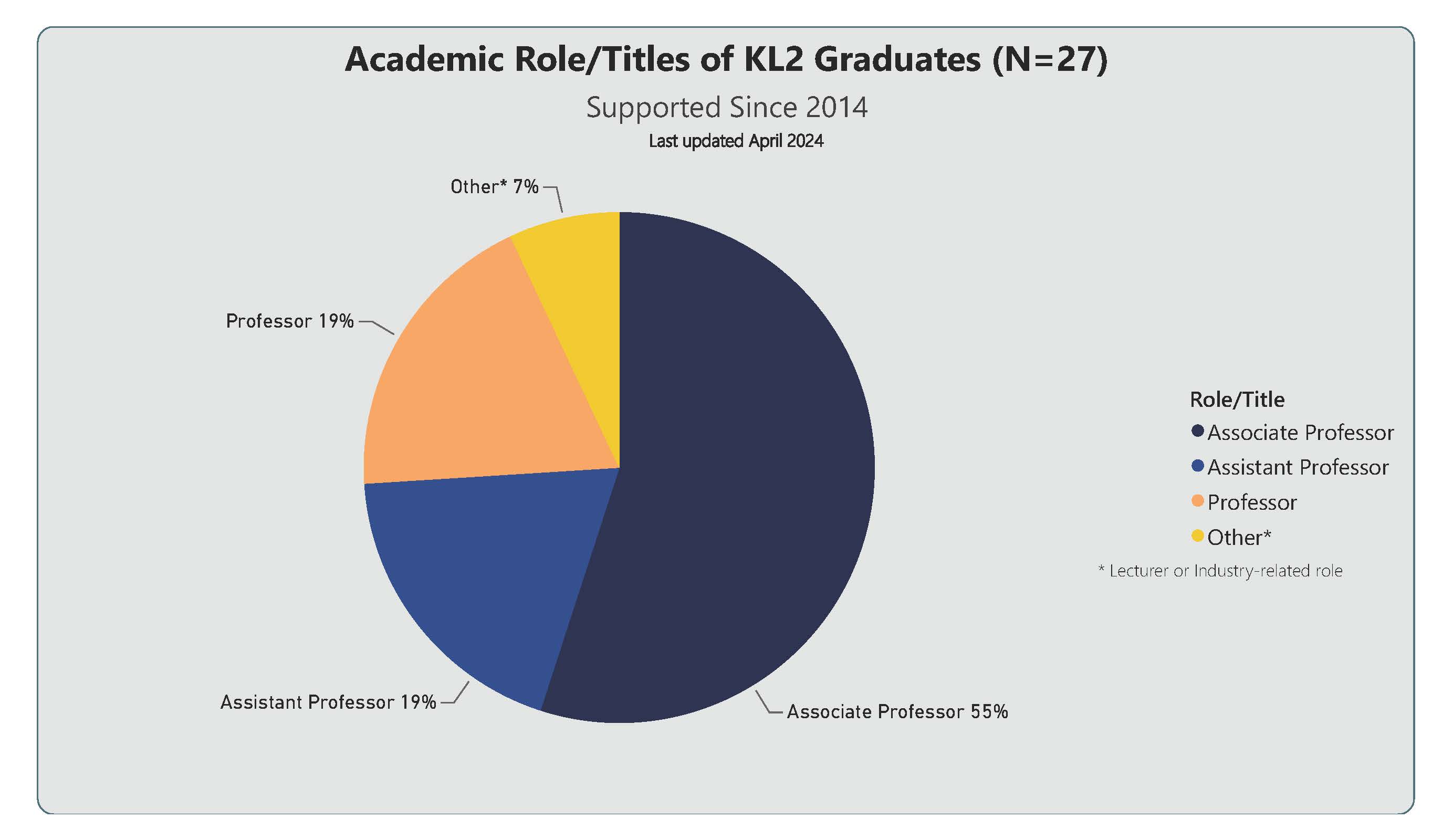
Academic Role/Titles of the 27 KL2 Graduates supported since 2014
Subsequent Grant Type for the 27 KL2 Graduates supported since 2014
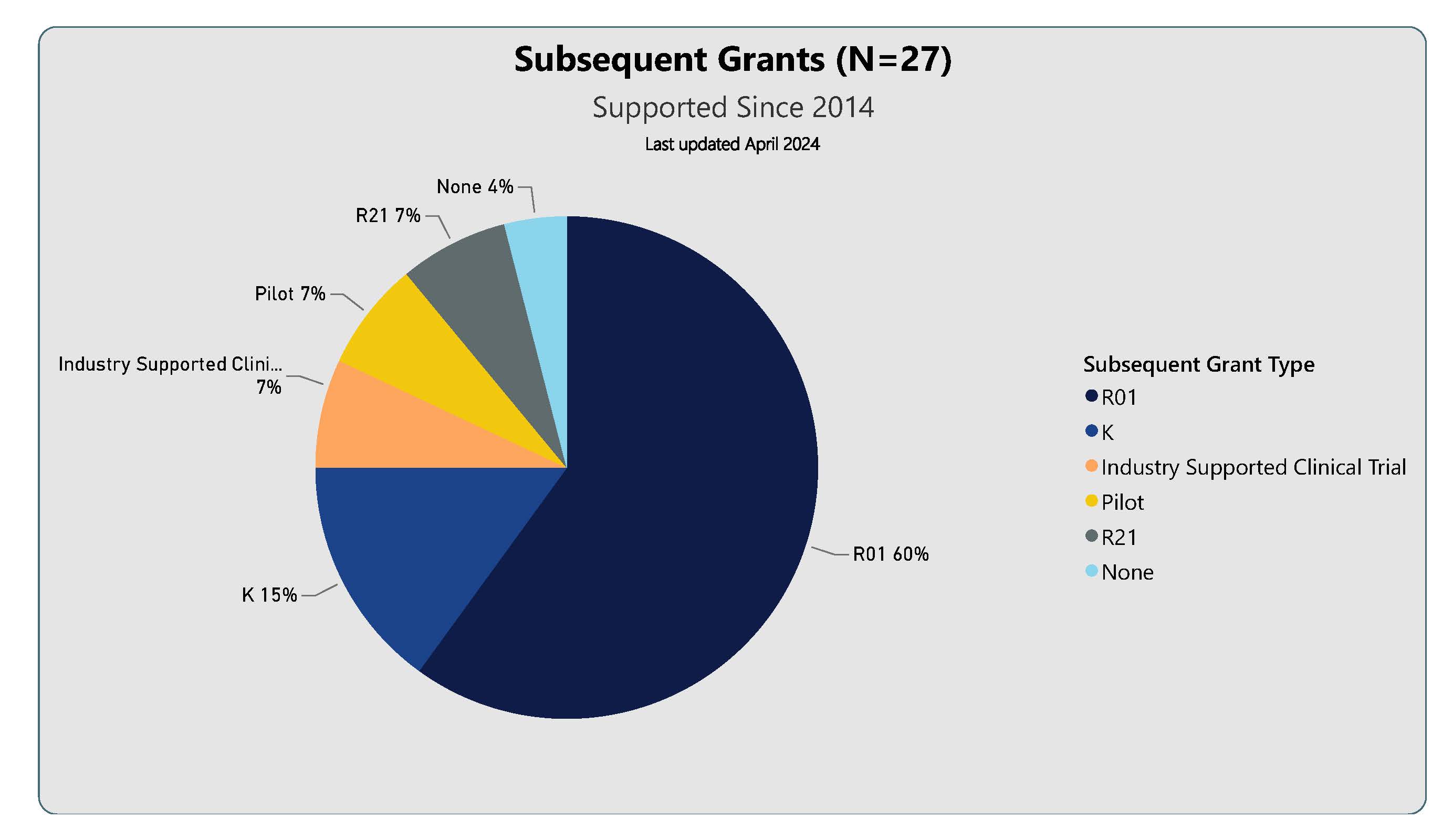
Clinical Protocols
Clinical Protocols
Center for Clinical Research
Leads: Mark Wallace, MD & Kathryn A. Gold, MD
ACTRI provided services to 988 investigators (i.e., >75% of active research faculty at UCSD), supported 2,784 clinical research studies (including > 60% of all of UCSD’s clinical trials) with the ACTRI Center for Clinical Research hosting over 7,000 visits annually for 17,508 unique study participants (25% UR, 48% women; n=4,021 for 2023). Of these, 373 services ($2,890,620) were delivered to our previous smaller group of partners (i.e., LJI, RCH, Salk Institute, SBP, and VASDHS).
Active ACTRI Clinical Protocols