Vision
From its urban core to its rural expanses and borderlands, greater San Diego comprises many communities facing complex health challenges. Our San Diego Clinical and Translational Science Award (CTSA) Hub connects communities through strategic partnerships, state-of-the-art resources, and transformative leadership. By advancing clinical and translational science (CTS), our hub will continue to be a national model for advancing science to improve health.
Mission
Our mission is to facilitate the research of others by providing resources, training, and collaboration opportunities for ACTRI scientists, health care providers, and the community.
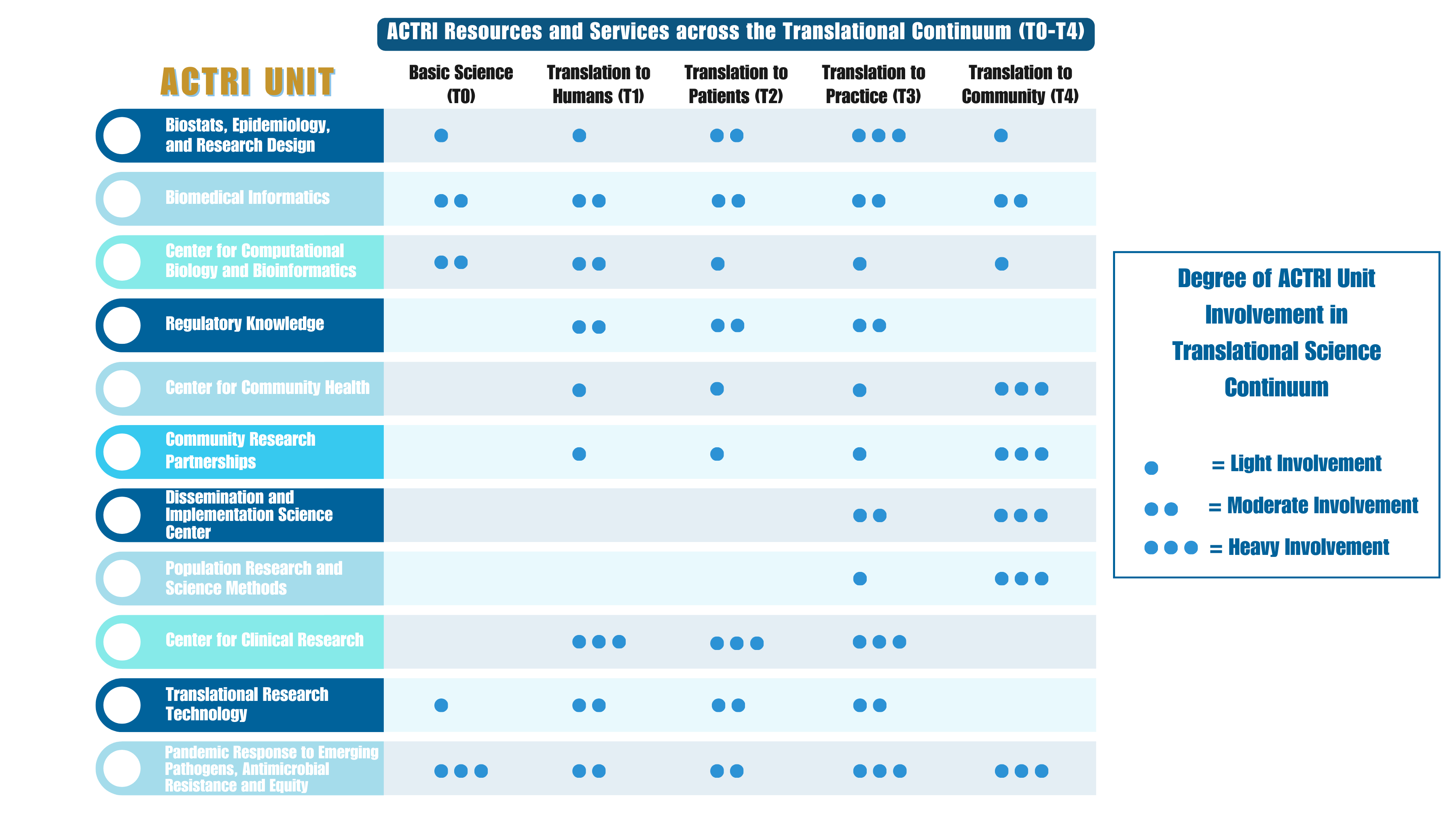
What is Clinical and Translational Science (CTS)?
ACTRI has transformed UCSD from a scientific environment focused on basic science into the translational powerhouse it is today. ACTRI aims to innovate across a broad range of clinical translational science (CTS) areas and integrate our research communities into our efforts.
CTS turns basic research into human benefits. Scientists test ideas in labs, starting with "basic research." Treatments are first tested in animals before human trials. Many experiments are needed before new medical devices or treatments are approved for regular use.
This science moves knowledge from labs to clinical and community settings, known as "bench-to-bedside" and "bedside-to-community" research. Success requires bi-directional communication among researchers, volunteers, patients, doctors, and communities.
Location & Contacts
Location & Contacts
ACTRI Office:
Email: ResearchComm@health.ucsd.edu
ACTRI Research Clinic:
Front Desk: (858) 534-1251
Mailing Address:
Altman Clinical and Translational Research Institute Building
9500 Gilman Drive, MC 0990
La Jolla, CA 92093-0990
Physical Address:
Altman Clinical and Translational Research Institute Building
9452 Medical Center Drive
La Jolla, CA 92037
Click here for larger, printable map
For help with this website, please submit your question here.
Become a Member
Join ACTRI for free in order to use the many services offered to researchers from UC San Diego, Salk Institute, La Jolla Institute for Immunology, El Centro Regional Medical Center, Mesa College, Sanford Burnham Prebys Medical Discovery Institute, Rady Children's Hospital, Eisenhower Health and the VA Medical Center.


