About Us
The ACTRI Workforce Development (WD) program creates a welcoming environment where clinical and translational science (CTS) careers launch and flourish. The ACTRI WD program implements novel initiatives across the career continuum, creates innovative and accessible training for CTS teams, improves retention of a workforce from different backgrounds and disciplines, and builds regional and national CTS capacity. Our WD program aims to:
-
Develop a skilled and collaborative CTS workforce to foster health innovation
-
Cultivate the CTS workforce through new partnerships and mentoring programs
-
Rigorously evaluate our Workforce Development
ACTRI is committed to developing the CTS workforce at every stage of their careers—from undergraduates to post doctoral researchers, high school students to faculty. This program strengthens students, post-docs and early career researchers' CTS skills as they learn from experienced faculty and biomedical industry professionals at UC San Diego.
Workforce Development Trainings and Activities
CREST
The ACTRI Clinical Research Enhancement through Supplemental Training (CREST) program, under the direction of Ravindra L. Mehta, MD, is designed to improve the skills and knowledge of clinician investigators and translational researchers by providing a comprehensive, cohesive learning experience in an environment that promotes interaction between basic scientists and clinical researchers.
Learn More about the CREST program
K12 Grant Support
The ACTRI K12 Program at UC San Diego is a mentored career development award funded by the National Center for Advancing Translational Sciences (NCATS). Designed for early-career faculty, the program provides up to three years of protected time for mentored research, grant writing, and career development, including coursework through ACTRI’s Clinical Research Enhancement through Supplemental Training (CREST) program. Scholars benefit from tailored mentorship and access to ACTRI’s robust translational research infrastructure. Alumni of the program have been highly successful in securing independent NIH funding (e.g., R01 awards) or transitioning to individual mentored K awards. The program aims to develop the next generation of translational scientists capable of accelerating the movement of research discoveries into clinical and community health settings, and is a core component of ACTRI’s Workforce Development Program, which supports CTS training across all career stages.
Mentoring and Career Development Resources
Effective mentoring is crucial to translational research. Below are key mentorship and career development resources at UC San Diego and beyond.
Individual Development Plan (IDP) & Mentor Compact
An Individual Development Plan (IDP) is recommended for all graduate students, postdocs, junior faculty, and research staff. NIH requires IDPs for funded trainees, and UC San Diego encourages their use. A Mentor Compact helps set clear expectations—access our modified mentor compact to tailor it to your mentorship needs.
Mentoring
For Faculty:- Faculty Mentoring Program – Roles & responsibilities of mentors and mentees.
- For general information about UC San Diego's faculty mentoring program, including roles and responsibilities of mentors and mentees, please visit Academic Affairs.
- UC San Diego Health Sciences National Center of Leadership in Academic Medicine (NCLAM) – A UCSD seven-month career development program for junior Health Sciences faculty.
- The UC San Diego Clinical Research Enhancement through Supplemental Training (CREST) and Master of Advanced Studies in Clinical Research (MAS) – Clinical research training for faculty involvement as trainees, mentors, or lecturers. Contact Maureen Eijsermans for details.
- Postdoc Resources – UC San Diego Office of Postdoctoral and Visiting Scholar Affairs offers IDP guidance and mentoring support.
- Career Connection – A free program for UC San Diego staff focused on career growth.
- Research Coordinator & Administrator Training (RCAT) – Monthly training on research compliance and best practices.
- Please contact the UC San Diego Health Science Research Compliance Program for more information.
- NIH Guide to Selecting a Research Mentor – Best practices for finding the right mentor.
- Faculty Mentor Program – Helps UC San Diego undergraduates secure research positions and present at the annual symposium. Contact Jessica Davis (jjd010@ucsd.edu).
UC San Diego ACTRI Education, Training, and Career Development Program – Provides consultations on integrating mentoring into training grants.
Additional External Mentoring Resources- University of Wisconsin-Madison – Entering Mentoring seminar and mentoring guides.
- Entering Mentoring: A Seminar to Train a New Generation of Scientists - geared toward mentors who wish to improve their skills.
- Resources for Each Phase of the Mentoring Relationship, - has links to information for both mentors and mentees.
- UCSF Clinical & Translational Science Institute – Free Mentor Development Program seminar and provides course materials for various aspects of mentorship are available free to the public.
Translational Science Career Development Seminar Series
A monthly series covering scientific communication, career planning, and research skills. Open to Early Career K awardees from UC San Diego and partner institutions.
Past Seminar Videos
Watch recorded sessions on the ACTRI YouTube Channel:
Clinical Research Coordinator (CRC) Training Program
The CRC Training Program provides a solid foundation for the roles and responsibilities of a clinical research coordinator. No prior experience is needed. Students will learn how to conduct clinical research. Students will be introduced to different types of human research studies.
Anticipating a call for CRC applications but timing is to be determined.
CreAting Dedicated Scientists through Research Education
The CreAting Dedicated Scientists through Research Education (CADRE) program is an initiative led by the UC San Diego Altman Clinical and Translational Research Institute (ACTRI) to provide research immersion opportunities for community college students in San Diego County. CADRE supports students in science, offering hands-on experience in translational research, financial assistance, and faculty mentorship. Partnering with San Diego Mesa community colleges and UC San Diego, the program empowers students to balance work and research, addressing challenges to participation while preparing them for scientific careers.
Through paid research positions, mentorship, and specialized training like the Clinical Coordinator Bootcamp, CADRE equips students with practical skills and professional development. Participants gain access to ACTRI’s resources, including vouchers for biostatistics consultations and other project support services. The program’s outcomes are transformative, with students pursuing advanced degrees, securing research positions, and contributing to science. CADRE exemplifies how education and mentorship can cultivate the next generation of scientific leaders.

Good Clinical Practice
What is Good Clinical Practice?
NIH issued a policy, effective January 1, 2017, establishing the requirement that all investigators and staff involved in the conduct, oversight, or management of NIH funded clinical trials must be trained in Good Clinical Practice (GCP).
GCP is an internationally recognized ethical and scientific quality standard for designing, conducting, recording, and reporting clinical trials that involve human participants. GCP ensures that the rights, safety, and well-being of trial participants are protected, consistent with the principles of the Declaration of Helsinki, and that the data generated in the trial is credible and reliable.
Why GCP is Important
- Participant Safety: It ensures the rights and well-being of participants are safeguarded.
- Data Quality: It guarantees the reliability and integrity of data, facilitating regulatory approval of new therapies.
- Global Standardization: GCP harmonizes clinical trial processes across different countries, allowing for international collaboration and recognition of research findings.
| Required Training | Who must complete the training? | When and how often must the training be completed? |
|---|---|---|
|
NIH issued a policy, effective January 1, 2017, establishing the requirement that all investigators and staff involved in the conduct, oversight, or management of NIH funded clinical trials must be trained in Good Clinical Practice (GCP). For additional information, see the NIH policy (Notice Number NOT-OD-16-148) and FAQ. GCP training can be completed through the CITI program. |
All investigators and staff involved in the conduct, oversight, or management of NIH funded clinical trials. |
All responsible parties should complete their GCP training before their involvement in the clinical trial. NIH requires that GCP training be renewed at least every 3 year. |
|
For questions on the UC San Diego implementation process, see the Research Compliance and Integrity website or contact Research Compliance and Integrity at rci@ucsd.edu, (858) 822-4939. |
||
Learn about UCSD's GCP policy and access GCP resources (UCSD AD login required)
Seminars
Slide decks and recordings of older seminars are on Pulse (UCSD AD login required)
Game Factory
About
The Game Factory creates interactive educational games for professionals, with a focus on game-based learning that is engaging, practical, and effective. Our games are designed to enhance real-world skills through immersive experiences that promote critical thinking and application of knowledge.
We’ve developed IRB Please, a game that helps improve the quality of IRB submissions by simulating the role of an IRB Analyst, and Clinical Guidance, which teaches the importance of using published clinical guidelines through realistic patient care scenarios. Each game is developed in collaboration with subject matter experts to ensure accuracy and relevance.
At The Game Factory, we believe learning should be active, enjoyable, and directly applicable to professional practice.
IRB Please
IRB Please is an interactive educational game designed to supplement the standard CITI (Collaborative Institutional Training Initiative) training for Institutional Review Board (IRB) writing and submission. Developed at UC San Diego’s Altman Clinical and Translational Research Institute (ACTRI), the game aims to enhance users’ practical understanding of the IRB review process by immersing them in the role of an IRB Analyst.
In the game, players are tasked with reviewing actual excerpts from real (de-identified) IRB submissions, evaluating whether each application meets the necessary ethical and procedural standards for approval. Players must carefully assess the completeness, accuracy, and compliance of the submitted documents, and then decide to approve, request revisions, or deny the application altogether.
IRB Please not only reinforces theoretical knowledge but also builds practical decision-making skills by simulating the challenges and nuances faced by IRB analysts in real-world settings. By engaging with authentic documentation and applying regulatory criteria, players gain a deeper appreciation for the critical role IRBs play in safeguarding human subjects in research. The game’s interactive format fosters active learning and provides immediate feedback, making it an effective tool for both novice researchers and experienced professionals seeking to sharpen their understanding of IRB requirements.
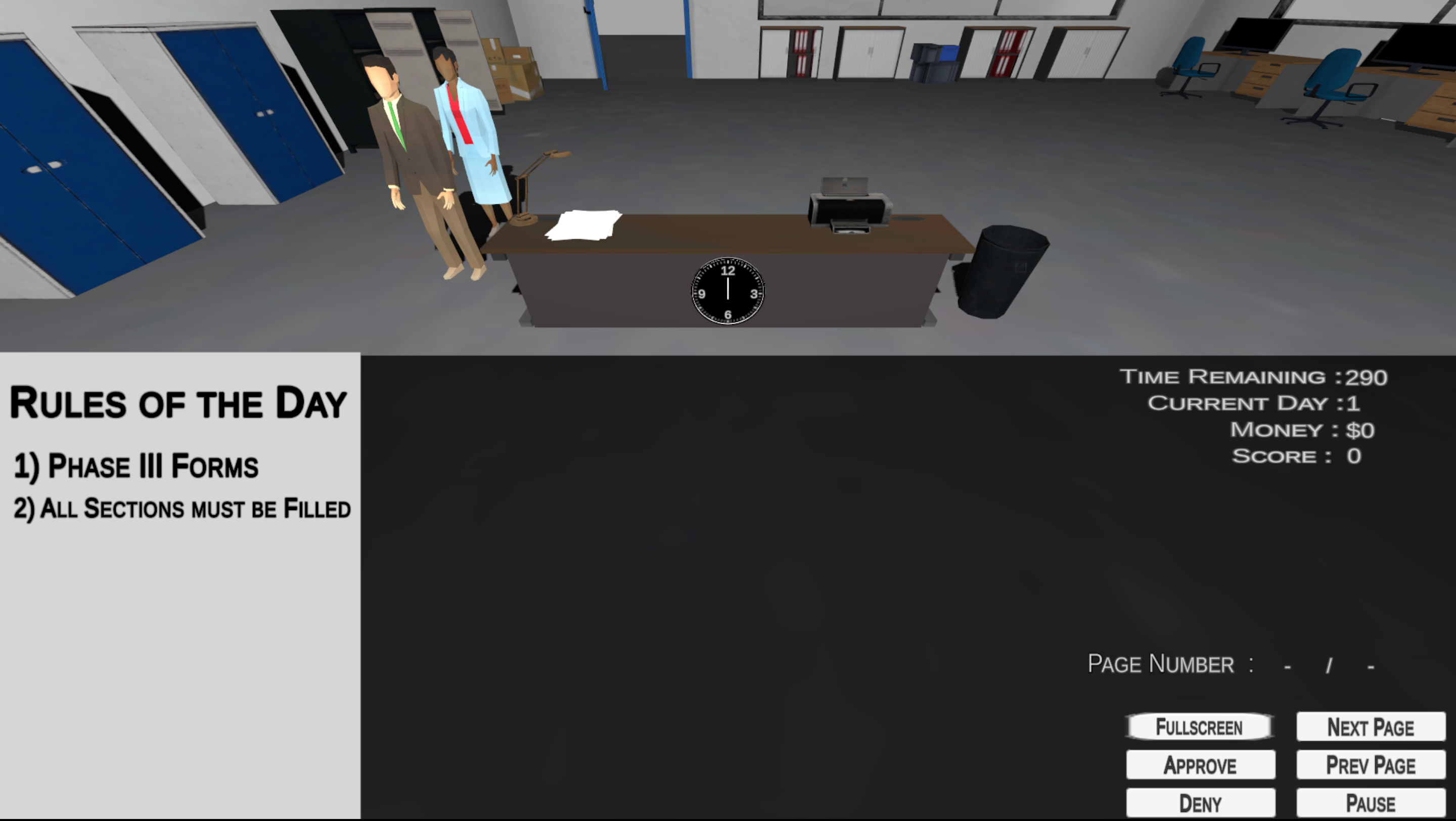
Clinical Guidance
Clinical Guidance is an interactive educational game designed to support dissemination and implementation science by demonstrating the real-world value of integrating evidence-based clinical guidelines into patient care. The game serves as a dynamic learning tool for clinicians and healthcare trainees, emphasizing the importance of following published protocols to improve patient outcomes and standardize care practices.
In Clinical Guidance, players take on the role of a newly practicing clinician navigating the complexities of real-world clinical decision-making. Through a series of simulated patient encounters, the player is responsible for conducting interviews, gathering patient histories and symptoms, forming diagnoses, and selecting appropriate treatments — all while referencing current clinical guidelines. The gameplay emphasizes critical thinking and the application of evidence-based knowledge in the context of varying patient presentations.
Following each case, players receive a targeted quiz that assesses their understanding of the patient’s condition, the chosen treatment plan, and the alignment of their decisions with established clinical guidelines. This reflective component reinforces learning by providing immediate feedback and encouraging players to consider how adherence to guidelines impacts patient safety, care quality, and overall health outcomes.
By blending clinical simulation with gamified learning, Clinical Guidance offers a compelling and engaging approach to professional development. It helps players internalize the process of guideline-based care delivery while fostering confidence in applying standardized protocols in diverse clinical scenarios. The game supports broader efforts to bridge the gap between research and practice, making it a valuable resource for medical education, continuing clinical training, and implementation science initiatives.
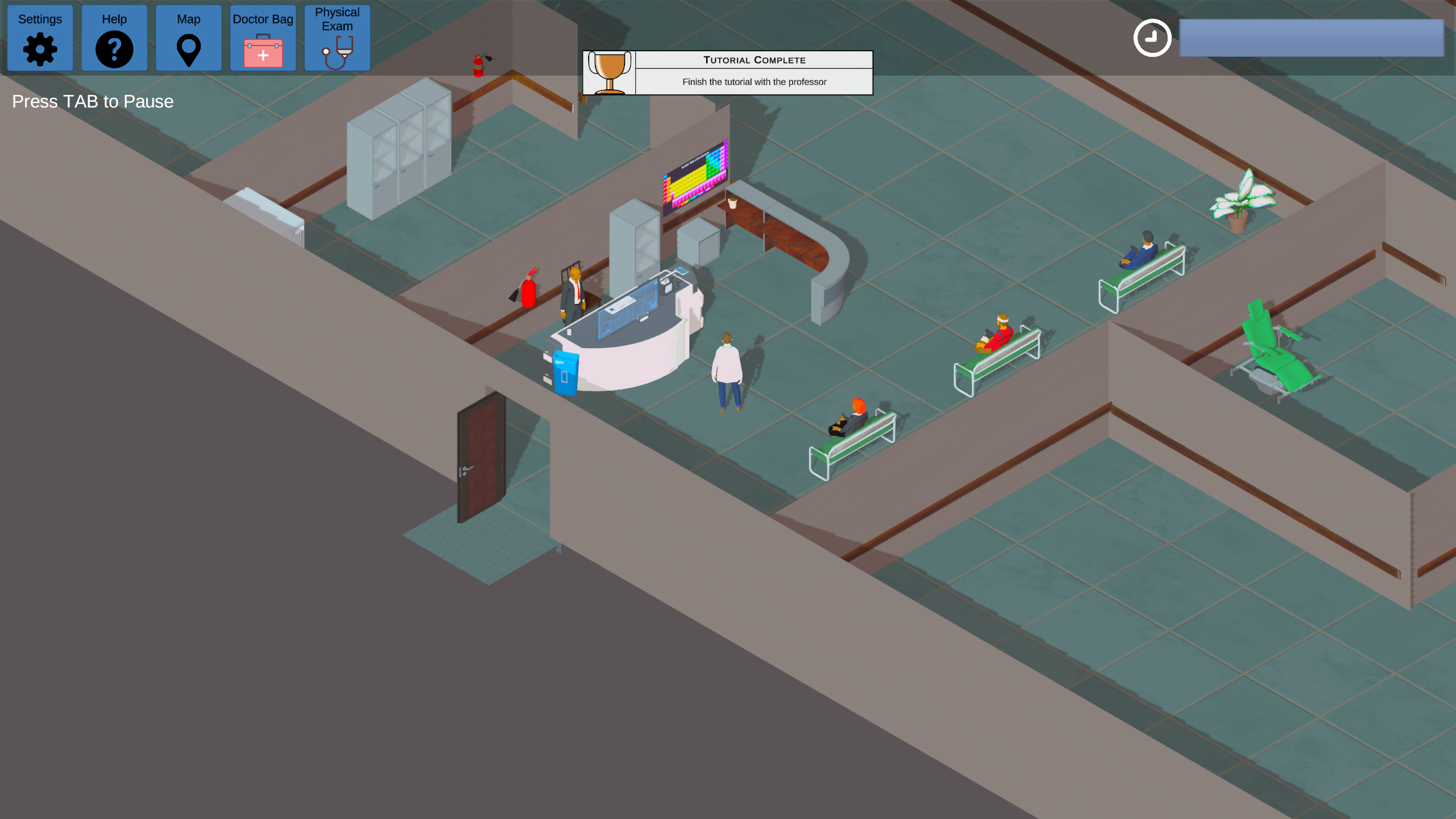
Contact the Workforce Development Program
Non-Discrimination Statement
In accordance with applicable Federal and State law and University policy, the University of California does not discriminate, or grant preferences, on the basis of race, color, national origin, religion, sex, disability, and/or other protected categories.
More information about Proposition 209 can be found here.
More information about the University of California Anti-Discrimination Policy can be found here.
